1st PUC Biology Question and Answer: BIOMOLECULES
Looking for 1st PUC Biology textbook answers? You can download Chapter 9: BIOMOLECULES Questions and Answers PDF, Notes, and Summary here. 1st PUC Biology solutions follow the Karnataka State Board Syllabus, making it easier for students to revise and score higher in exams.
Karnataka 1st PUC Biology Textbook Answers—Reflections Chapter 9
BIOMOLECULES Questions and Answers, Notes, and Summary
1st PUC Biology Chapter 9
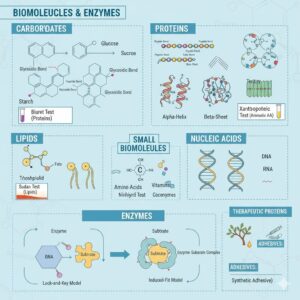
BIOMOLECULES
Scroll Down to Download BIOMOLECULES PDF
Question and Answer:
Question 1.
What are macromolecules? Give examples.
Answer:
Biomacromolecules are large polymeric compounds found in the acid-insoluble fraction of the cell. They have a high molecular weight, usually more than 1000 Daltons. Examples include polysaccharides, proteins, and nucleic acids.
Question 2.
Illustrate a glycosidic, peptide and a phospho-diester bond.
Answer:
- Glycosidic bond – It is a covalent bond formed between two monosaccharide molecules by the removal of a water molecule (condensation). Example: In maltose, the bond is formed between carbon-1 of one glucose and carbon-4 of another glucose.
- Peptide bond – It is a covalent bond formed between the carboxyl group (–COOH) of one amino acid and the amino group (–NH₂) of another amino acid with the release of a water molecule. Example: In proteins, peptide bonds link amino acids in chains.
- Phosphodiester bond – It is a covalent bond formed between the hydroxyl group (–OH) of the 3’ carbon of one nucleotide’s sugar and the phosphate group attached to the 5’ carbon of the adjacent nucleotide. This bond joins nucleotides together in nucleic acids like DNA and RNA.
Question 3.
What is meant by tertiary structure of proteins?
Answer:
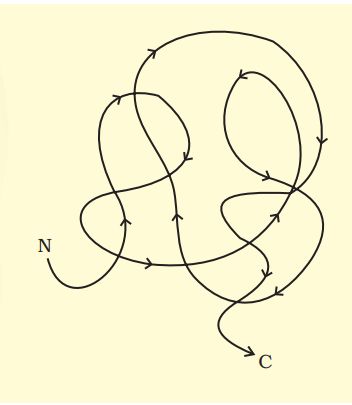
In proteins, only right-handed helices are observed as part of the secondary structure. In addition, the long protein chain folds upon itself like a hollow woolen ball, giving rise to the tertiary structure. This folding provides a three-dimensional view of the protein. The tertiary structure is absolutely necessary for the many biological activities of proteins. (Fig. 9.2 a, b)
Question 4.
Find and write down structures of 10 interesting small molecular weight biomolecules. Find if there is any industry which manufactures the compounds by isolation. Find out who are the buyers.
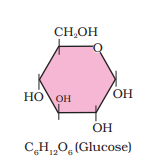
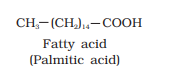
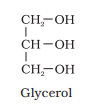
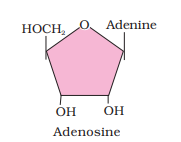
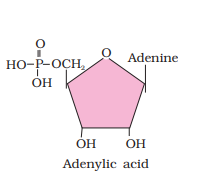
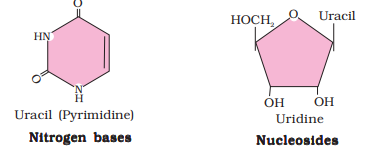
Ranboxy, Merck, etc. manufacture these macromolecules. Research institutes by these micromolecules
Question 5.
Proteins have primary structure. If you are given a method to know which amino acid is at either of the two termini (ends) of a protein, can you connect this information to purity or homogeneity of a protein?
Answer:
Yes. The primary structure of proteins refers to the linear sequence of amino acids in a polypeptide chain, with a free amino group at the N-terminus and a free carboxyl group at the C-terminus.
If a method can identify the amino acid present at either end of the chain, the result should always be the same for a pure and homogeneous protein sample. If more than one type of amino acid is detected at a terminus, it indicates that the protein sample is either impure (contaminated with other proteins) or heterogeneous (a mixture of different polypeptides).
Question 6.
out and make a list of proteins used as therapeutic agents. Find other applications of proteins (e.g., Cosmetics etc.)
Answer:
Therapeutic Agents:
- Contraceptive pills: These are hormonal preparations, made up of proteins.
- Nutritional supplements: Many brands are available as protein supplements.
Example:
Other Applications:
- Chicken cubes are used in making soups and dishes.
- Proteins are also used in cosmetics (collagen, keratin), food industry (gelatin, casein), and as enzymes in detergents.
Question 7.
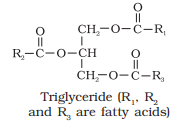
Explain the composition of triglyceride.
Answer:
Glycerol combines with three similar fatty acids to form triglyceride. Structure/Composition of triglyceride
(i) O
||
CH2 – O – C – R1
(ii) O
||
R2 – C – O – CH
(iii) O
||
CH2 – O – C – R3
Question 8.
Can you describe what happens when milk is converted into curd or yoghurt, from your understanding of proteins.
Answer:
When milk is converted into curd or yoghurt, the milk protein casein undergoes coagulation. During this process, casein is transformed into paracasein, forming a semi-solid gel-like structure that gives curd or yoghurt its texture. The coagulation is primarily caused by the acidification of milk by lactic acid produced by bacteria.
Question 9.
Can you attempt building models of biomolecules using commercially available atomic models (Ball and Stick models).
Answer:
Yes, biomolecules can be modeled using commercially available atomic models such as Ball-and-Stick kits. These kits allow us to physically represent atoms as balls and bonds as sticks, helping to visualize the 3-dimensional structure of molecules.
For example:
- Amino acids can be built showing the central carbon, amino group, carboxyl group, and side chain.
- Simple sugars like glucose can be modeled to show ring structures.
- Nucleotides can be assembled to visualize bases, sugar, and phosphate groups.
Building these models helps in:
- Understanding molecular geometry.
- Studying bond angles and connectivity.
- Demonstrating isomerism and stereochemistry.
Question 10.
Attempt titrating an amino acid against a weak base and discover the number of dissociating (ionizable) functional groups in the amino acid.
Answer:
Yes, an amino acid can be titrated against a weak base (like NaOH) to determine the number of ionizable functional groups. Here’s how it works:
- Principle:
- Amino acids have at least two ionizable groups: the amino group (-NH₃⁺) and the carboxyl group (-COOH). Some amino acids have additional ionizable side chains.
- During titration, these groups lose protons at different pH values, leading to buffer regions in the titration curve.
- Procedure:
- Dissolve the amino acid in water.
- Titrate with a standard solution of weak base (e.g., NaOH).
- Monitor pH after each addition using a pH meter or indicator.
- Observation:
- The titration curve shows plateaus at pH values where ionization occurs.
- Each plateau corresponds to an ionizable group.
- Analysis:
- Count the number of plateaus/inflection points to determine the number of dissociating groups.
- For example:
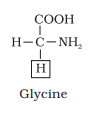
- Glycine: 2 ionizable groups (α-carboxyl and α-amino).
- Aspartic acid: 3 ionizable groups (α-carboxyl, α-amino, β-carboxyl).
This experiment helps determine pKa values and acid-base behavior of amino acids.
Question 11.
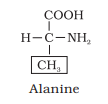
Draw the structure of the amino acid, alanine.
Answer:
Structure of alanine
COOH
|
H — C —NH2
|
CH3
Question 12.
What are gums made of? Is Fevicol different?
Answer:
Gums are natural plant-derived substances composed mainly of polysaccharides and lignocellulose, secreted from resin ducts of plants. They are adhesive in nature and can increase the viscosity of solutions, making them useful in food, pharmaceuticals, and adhesives.
Fevicol, on the other hand, is different. It is a synthetic adhesive made from polyvinyl acetate (PVA) or similar polymers. Unlike natural gums, Fevicol hardens as the solvent evaporates and is mainly used for household or industrial bonding. It is artificial and chemically engineered, whereas gums are naturally occurring.
Question 13.
Find out a qualitative test for proteins, fats and oils, amino acids and test any fruit juice, saliva, sweat and urine for them.
Answer:
(a) Test for Proteins
- Xanthoproteic Test: Add concentrated nitric acid to the sample. Yellow color indicates aromatic amino acids (protein presence).
- Biuret Test: Add 1% CuSO₄ followed by 10% NaOH. Violet or purple color indicates proteins.
(b) Test for Amino Acids
- Ninhydrin Test: Add Ninhydrin reagent and heat gently. Purple or bluish color indicates free amino acids.
(c) Test for Fats and Oils
- Sudan III Test: Add Sudan III stain. Red-stained droplets indicate fats.
- Spot Test: Rub a drop of sample on filter paper. Grease spot indicates fat.
Question 14.
Find out how much cellulose is made by all the plants in the biosphere and compare it with how much of paper is manufactured by man and hence what is the consumption of plant material by man annually. What a loss of vegetation!
Answer:
- Cellulose Production by Plants:
- Cellulose is the main component of plant cell walls.
- Globally, plants produce about 100 billion tons of cellulose per year through photosynthesis. This is part of the total biomass generated annually.
- Paper Production by Humans:
- Worldwide paper production is roughly 400 million tons per year.
- Paper is mainly made from wood pulp, which contains cellulose.
- Comparison & Consumption:
- Humans use about 4 billion tons of plant cellulose for paper, compared to 100 billion tons produced naturally.
- This means human consumption of plant material for paper is less than 1% of total cellulose produced naturally, but it still causes deforestation, habitat loss, and vegetation degradation.
- Loss of Vegetation:
- Though small in percentage, the destruction of forests for paper, furniture, and other uses contributes significantly to loss of biodiversity and ecological balance.
- The impact is higher locally in forest-rich regions.
Conclusion:
While plants produce vast amounts of cellulose, human consumption—even if comparatively small—is concentrated in specific areas, leading to significant vegetation loss and ecological impact.
Question 15.
Describe the important properties of enzymes.
Answer:
The important properties of enzymes are:
- Lowering the activation energy:Enzymes reduce the minimum energy required for a reaction to occur.
- Stabilizing the transition state:Enzymes lower the energy of the transition state, making reactions faster.
- Providing an alternative pathway:Enzymes offer a reaction pathway that requires less energy.
- Reducing reaction entropy change:They help organize substrates to reduce randomness during reactions.
- Temperature effect:Moderate increases in temperature accelerate enzyme-catalyzed reactions.
- Specificity:Each enzyme acts on a specific substrate; “one enzyme, one substrate.”
- Efficiency:A small quantity of enzyme can catalyze a large number of reactions.
Additional Question and Answer
Question 1.
What is the difference between a coenzyme and a prosthetic group?
Answer:
- Coenzyme: A coenzyme is an organic molecule that temporarily binds to an enzyme to assist in catalysis. They are often derived from vitamins and are not permanently attached. Example: NAD⁺ (Nicotinamide adenine dinucleotide).
- Prosthetic group: A prosthetic group is a non-protein component that is permanently bound to an enzyme and is essential for its activity. Example: Heme in hemoglobin.
Question 2.
Explain the lock-and-key and induced-fit models of enzyme action.
Answer:
- Lock-and-key model: The enzyme’s active site is a perfect fit for the substrate, like a key fitting into a lock.
- Induced-fit model: The enzyme undergoes a conformational change to fit the substrate more snugly when the substrate binds, enhancing catalysis.
Question 3.
Differentiate between saturated and unsaturated fats.
Answer:
- Saturated fats: Contain no double bonds between carbon atoms; usually solid at room temperature. Example: Butter.
- Unsaturated fats: Contain one or more double bonds; usually liquid at room temperature. Example: Olive oil.
Question 4.
What is the role of vitamins as biomolecules?
Answer:
- Vitamins are organic biomolecules required in small quantities for normal metabolism.
- They act as coenzymes or precursors for coenzymes in enzymatic reactions.
- Example: Vitamin B1 (Thiamine) → Coenzyme TPP for decarboxylation reactions.
Click Here to Downlaod BIOMOLECULES PDF Notes
Click Here to Watch BIOMOLECULES Video